Order of any reaction can be measured with respect to each of the reactants in the reaction. Third Order ReactionWhen the rate of the reaction depends on the third power of the concentration term in the rate equation then the reaction is called as the third order reaction. The rate constant is a proportionality constant where the rate of reaction that is directly correlated to the concentration of the reactant.
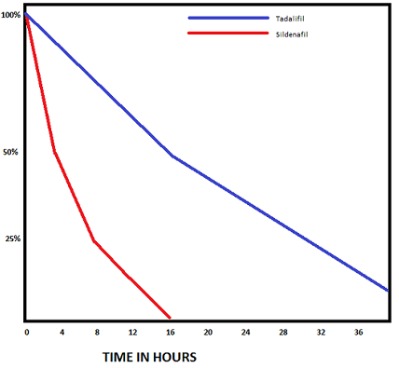
Reaction of shifting orderOrder of a reaction is an experimentally determined quantity. Zero order reactionOrder of a reaction is an experimentally determined quantity.
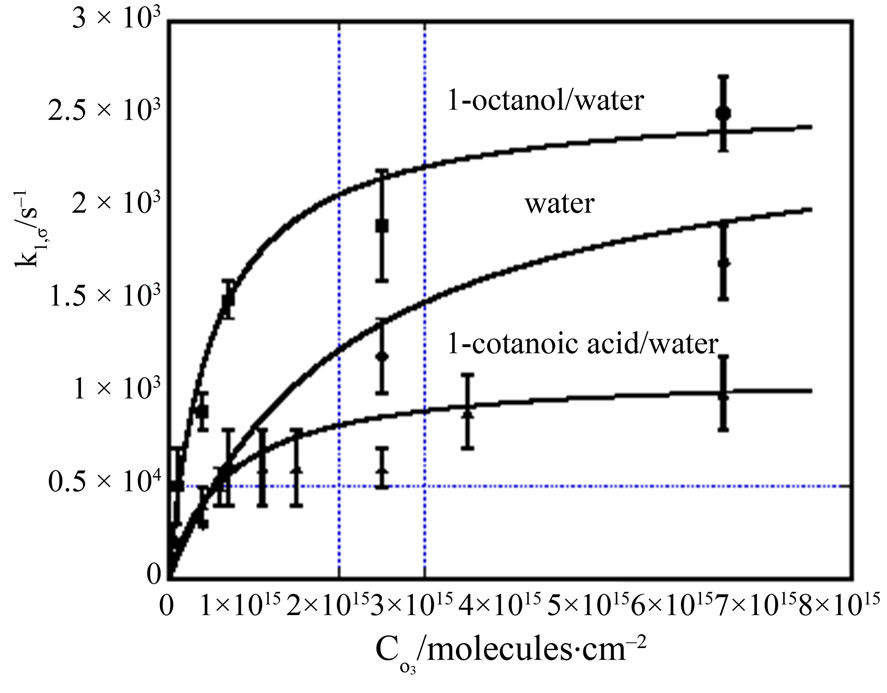
First order reactionOrder of a reaction is an experimentally determined quantity. Pseudo first order reactionOrder of a reaction is an experimentally determined quantity.

The kinetics of the reaction between vitamin C (-ascorbic acid) and ferric chloride hexahydrate was investigated in acidic medium at pH 3 spectrophotometrically. Stuck with derivation of the integrated rate equation for a pseudo first order equilibrium reaction322Conc. The integrated rate law for a first-order reactionis a common example of the law of exponential change. Can SN1 reactions be considered as pseudo unimolecular reactions The present book is included for the students of higher secondary secondyear.
First order reactionFor a first order reaction the half-life depends only on the rateconstant:1sthalflife. The particles on one side of the surface are A and on the other side are B. The overall molecularity of a complex reaction has no significance.
Inga kommentarer:
Skicka en kommentar
Obs! Endast bloggmedlemmar kan kommentera.